
Overview
Respiratory syncytial virus (RSV) is a common respiratory virus that infects the nose, throat, lungs, and breathing passages. It circulates broadly alongside other seasonal viruses, including influenza. RSV can be serious, especially for premature and very young infants (≤6 months), people with chronic heart or lung disease, people who are immunocompromised, and older adults (65+). While these groups are at increased risk for severe disease, the potential for complications is a concern for all age groups.
The National Foundation for Infectious Diseases (NFID) supports RSV education by engaging key audiences, sharing firsthand experiences of RSV among all age groups, and providing resources to help raise awareness of the burden of disease. In January 2022, NFID issued a Call to Action outlining key strategic priorities to reduce the burden of RSV across the lifespan. The Call to Action was based on discussions from an NFID-led virtual roundtable held with a multidisciplinary group of stakeholders and subject matter experts to review US public health priorities for the prevention and treatment of RSV.
Since the initial publication of the Call to Action, there have been advancements in the development of potential interventions and additional disease surveillance, as well as concerning trends in RSV activity in the US in 2022-2023.
The goals of this RSV progress report are to:
- Reaffirm strategies to address unmet needs and issues
- Provide an update on progress and new developments in the US
- Highlight future considerations
Call to action: Issues and Priorities
This is an optimal time to drive progress for RSV surveillance, prevention, diagnosis, and treatment.
Challenges and Strategic Priorities
| Challenges | Strategic Priorities |
| Pandemic-related shifts in focus | Increase RSV awareness |
| Lagging surveillance efforts | Strengthen public health responses to RSV |
| Current limitations of RSV prevention | Prepare for implementation of new RSV interventions, as approved |
| Gaps in diagnostic testing |
Updates and New Developments
Progress Made and Ongoing Challenges
2022-2023 Disease Activity in the US
Like seasonal influenza (flu) and other respiratory viruses, RSV is equally unpredictable and disease activity can vary by region. Historically, RSV season started in the fall and peaked in the winter months. However, in the last two years, the virus began circulating in the summer months in specific regions of the US, kicking off heightened disease activity that extended through the fall, and into the winter.
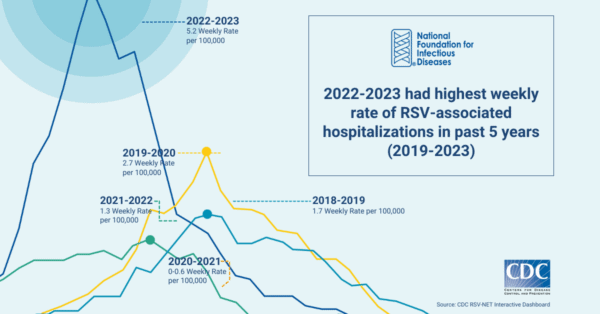
This surge in RSV activity coincided with an increase in COVID-19 and influenza activity to create an unprecedented strain on US hospitals and emergency rooms. In addition to the increased RSV activity, the 2022-2023 US respiratory season also reported the highest levels of influenza activity since the height of the pandemic. Many emergency departments across the country were forced to adopt measures similar to those used during previous, severe surges of COVID-19.
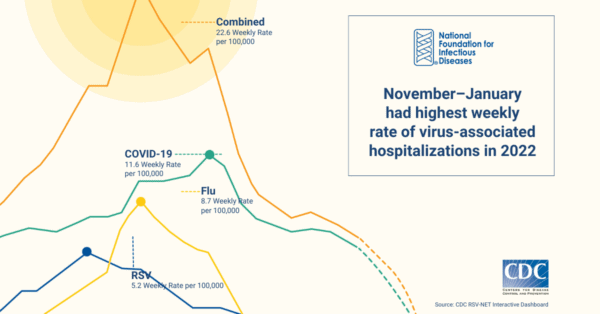
A lack of access to rapid, accurate, and inexpensive diagnostics impedes understanding of disease activity and burden. As noted in surveillance by the Centers for Disease Control and Prevention (CDC), these figures likely underestimate the actual rates of RSV, as the rates are based on those who tested positive for RSV through a laboratory-confirmed test ordered by a healthcare professional. Without further healthcare professional education and an increase in testing, the data remain incomplete. Although data are needed for public health purposes, challenges will remain until testing directly impacts clinical care.
Advancements Supporting Strategic Priorities
Since publication of the initial NFID Call to Action, progress has been made in several key areas.
Increased CDC surveillance. During the 2022-2023 respiratory season, CDC released two new dashboards providing integrated data on COVID-19, influenza, and RSV hospitalizations in the US, which are both updated weekly:
- Respiratory Virus Hospitalization Surveillance Network (RESP-NET) interactive dashboard brings together data for laboratory-confirmed COVID-19, influenza, and RSV-associated hospitalizations among children and adults. This dashboard allows public health experts to compare hospitalization rates for the three viruses in different demographic groups—ultimately helping stratify risk and drive informed, effective vaccination and treatment policies.
- National Emergency Department Visits for COVID-19, Influenza, and Respiratory Syncytial Virus dashboard focuses on emergency department visits associated with COVID-19, influenza, and RSV, using data tracked by the National Syndromic Surveillance Program (NSSP). As data and features for this dashboard expand, public health officials will be able to track symptoms of emergency department patients and monitor virus levels for signs of unusual increases or potential outbreaks.
New interventions for older adult, maternal, and pediatric populations. New interventions are in development to prevent RSV, including vaccines and monoclonal antibodies targeting vulnerable populations (infants/young children, older adults, pregnant women, and people with compromised immune systems). If approved by the US Food and Drug Administration (FDA) and recommended by CDC, these interventions will be critical to improved disease prevention approaches for future respiratory seasons.
Reduced barriers to access via lower costs. The 2022 Inflation Reduction Act went into effect on January 1, 2023. The Part D Improvements section includes the elimination of out-of-pocket payments for all recommended adult vaccines by Medicare patients. Reducing this financial burden for older adults will help increase access to vaccination and support public health measures for disease prevention.
Future Considerations
Pathways to Reach the Broadest Population and Build Health Equity
Further action is required to mitigate challenges and build on progress made to date. During the NFID roundtable, stakeholders discussed strategies to capitalize on emerging opportunities for improved prevention, diagnosis, treatment, and surveillance of RSV. These remain relevant and urgent pathways to reducing the burden of RSV and addressing RSV-related disparities.
Public Health Capacity
- Educate key stakeholders to build understanding of RSV and its impact across the lifespan, along with support for new interventions. These efforts should include expanded outreach and education for internists and healthcare professionals who routinely care for older adults, along with ongoing outreach to reach pediatric healthcare professionals, parents, and pregnant women. Strategies may include digital and traditional media awareness campaigns, consumer and patient educational resources, and innovative digital storytelling campaigns to reach and educate parents of young children as well as older adults and their caregivers.
- Expand surveillance and diagnostics to guide US public health response and facilitate treatment for patients. At the federal level, priority should be placed on establishing a multi-year baseline of surveillance data to inform public health guidelines and investments, and to monitor the impact on disease transmission and outcomes over time. It is also important to ensure state and local health departments have adequate dedicated resources and the technical support necessary to conduct routine RSV surveillance in their jurisdictions.
For diagnostics, a combination of actions is needed: creating more convenient and affordable diagnostic tests; closing gaps in insurance coverage; and addressing logistical and procedural barriers, including delayed or incomplete reporting of test results. Education will also be important to increase the uptake of available diagnostic tests, as new treatment options become available.
US Public Health Recommendations and Implementation
- Pursue broad public health recommendations for evidence-based interventions that will be beneficial on a population basis. Recent evidence and experience—with influenza, COVID-19, and other vaccines—provide a compelling argument that broad, age-based recommendations can maximize uptake. While narrower, risk-based recommendations may be appropriate in certain circumstances, CDC and other decision-making bodies should seek the broadest appropriate application of new interventions supported by clinical and economic evidence.
- Address implementation and payment issues to best support the introduction and rollout of interventions under review to ensure equitable access and maximize public health benefit in time for the 2023-2024 respiratory season. This may include questions related to payment, systems integration, adverse event reporting, electronic medical record tracking, and other relevant areas.
- Identify funding and payment sources for preventive interventions. This will require reliable payment beyond traditional health insurance to ensure first-dollar coverage. For example, the CDC Vaccines for Children (VFC) program is critical for ensuring access to vaccines and other interventions in children of under- or uninsured families. Additional potential barriers will need to be removed, including cost-sharing requirements for recommended vaccines for adults covered under Medicaid. Further changes to Medicare may be needed to ensure adequate compensation for costs associated with administering preventive RSV interventions, and Current Procedural Terminology (CPT®) codes will need to be available immediately to support payment and reporting.
Conclusion
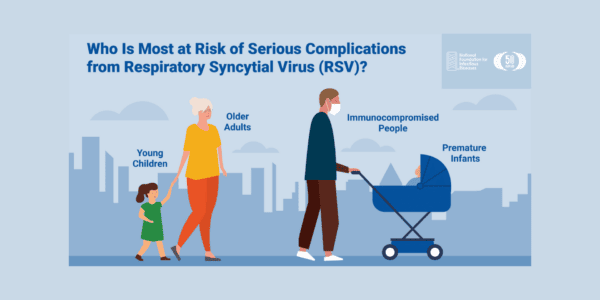 New and innovative RSV interventions have the potential to significantly reduce the burden of RSV in young children and older adults in the US. Successful implementation of these interventions will rely on raising awareness about RSV and the burden of disease, and on appreciation of the benefits of prevention. There is a need for ongoing RSV education and disease awareness, in support of the implementation of new interventions, along with continued actions to improve equity and access.
New and innovative RSV interventions have the potential to significantly reduce the burden of RSV in young children and older adults in the US. Successful implementation of these interventions will rely on raising awareness about RSV and the burden of disease, and on appreciation of the benefits of prevention. There is a need for ongoing RSV education and disease awareness, in support of the implementation of new interventions, along with continued actions to improve equity and access.
NFID remains committed to ongoing education and engagement, and will update the Progress Report: Reducing the Burden of RSV across the Lifespan on a seasonal basis to reflect disease activity as well as related approvals and recommendations from US regulatory bodies.
GSK, Johnson & Johnson Health Care Systems, Inc., Merck & Co., Inc., Pfizer Inc., and Sanofi Pasteur Inc. provided funding for this activity. NFID policies restrict funders from controlling program content
References
Centers for Disease Control and Prevention. RSV Trends and Surveillance. Accessed December 20, 2021. www.cdc.gov/rsv/research/us-surveillance.html.
Centers for Disease Control and Prevention. RSV in Older Adults and Adults with Chronic Medical Conditions. Accessed December 20, 2021. www.cdc.gov/rsv/high-risk/older-adults.html.
Centers for Disease Control and Prevention. Respiratory Syncytial Virus Infection (RSV). Accessed December 20, 2021. www.cdc.gov/rsv/index.html.
Rha B, Curns AT, Lively JY, et al. Respiratory Syncytial Virus-Associated Hospitalizations Among Young Children: 2015-2016. Pediatrics. 2020;146(1):e20193611. doi:10.1542/peds.2019-3611.
Centers for Disease Control and Prevention. Increased Interseasonal Respiratory Syncytial Virus (RSV) Activity in Parts of the Southern United States. June 10, 2021. Accessed December 20, 2021. https://emergency.cdc.gov/han/2021/han00443.asp.
Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179-186. doi:10.1001/jama.289.2.179.
Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360(6):588-598. doi:10.1056/NEJMoa0804877.
Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749-1759. doi:10.1056/NEJMoa043951.
Hasegawa K, Tsugawa Y, Brown DFM, Mansbach JM, Camargo CA. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics. 2013;132(1):28-36. doi:10.1542/peds.2012-3877.
Amand C, Tong S, Kieffer A, Kyaw MH. Healthcare resource use and economic burden attributable to respiratory syncytial virus in the United States: a claims database analysis. BMC Health Serv Res. 2018;18(1):294. doi:10.1186/s12913-018-3066-1.
Updated April 2023
Related Resources
Contagious Chronicles: Don’t Be A Dreaded Spreader
In this episode, NFID experts offer insights on updated recommendations from the Centers for Disease Control and Prevention (CDC) to help prevent the spread of respiratory viruses, including COVID-19, influenza (flu), and respiratory syncytial virus (RSV)

Contagious Chronicles: Updates on COVID-19, RSV, and More
In the inaugural episode, NFID experts discuss new COVID-19 vaccine recommendations for older adults and other updates from the February 2024 meeting of the Advisory Committee on Immunization Practices (ACIP) …

RSV Social Media Graphics
Share these graphics to help raise awareness about RSV prevention and treatment across the lifespan
