July celebrations include Independence Day and National Fireworks Safety Month. With both independence and safety in mind, it is also a good time to reflect upon the in-depth and independent review process all vaccines must go through in order to be licensed and considered safe.
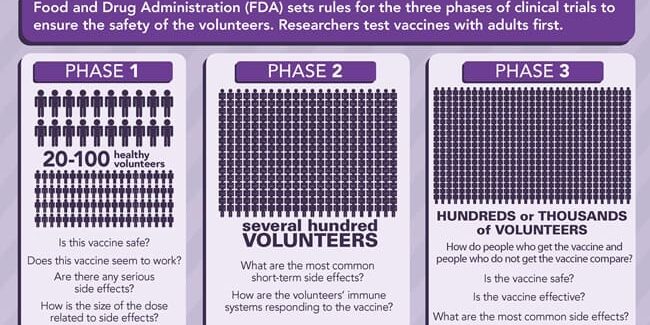
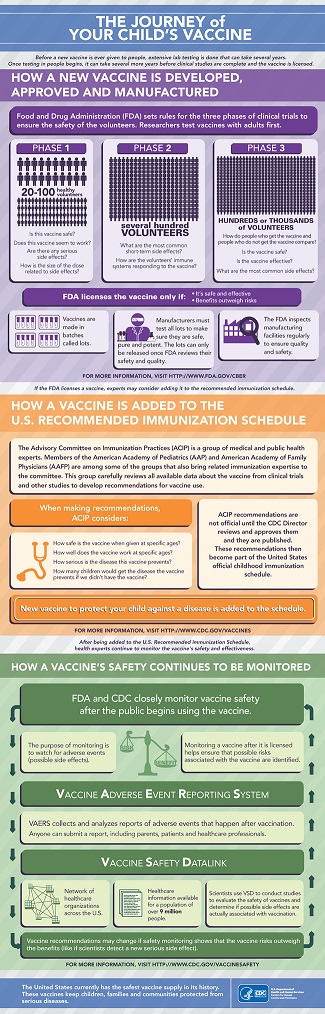 The US Food and Drug Administration (FDA) is responsible for ensuring the safety, effectiveness, and availability of vaccines. Before the FDA licenses a vaccine, it is tested extensively by the manufacturer. Next, FDA scientists and medical professionals carefully evaluate all available information about the vaccine to determine its safety and effectiveness. Vaccines that are being developed for children are first tested in adults and typically involve three phases of clinical trials. In many cases, it can take 10-15 years from the development of a vaccine to licensure by FDA.
The US Food and Drug Administration (FDA) is responsible for ensuring the safety, effectiveness, and availability of vaccines. Before the FDA licenses a vaccine, it is tested extensively by the manufacturer. Next, FDA scientists and medical professionals carefully evaluate all available information about the vaccine to determine its safety and effectiveness. Vaccines that are being developed for children are first tested in adults and typically involve three phases of clinical trials. In many cases, it can take 10-15 years from the development of a vaccine to licensure by FDA.
Once a vaccine has been licensed by FDA, the Advisory Committee on Immunization Practices (ACIP) reviews the trial data and makes a recommendation for vaccine use. The ACIP recommendations are then reviewed and approved by the Director of the Centers for Disease Control and Prevention (CDC) Director and the US Department of Health and Human Services, followed by publication in the CDC Morbidity and Mortality Weekly Report, at which time they become part of the recommended immunization schedule. Once a vaccine is added the recommended schedule, monitoring for adverse events is essential, as even large clinical trials may not be big enough to reveal rare side effects. Additionally, clinical trials may not include groups who may have a higher risk of side effects such as pregnant women or older adults. Meanwhile, vaccine safety is monitored through the Vaccine Adverse Event Reporting System and the Vaccine Safety Datalink.
The independent and multi-layered vaccine review process can be complicated but is in place to ensure that vaccines are held to the highest standards of safety. Moreover, continuous monitoring of health problems after vaccination ensures that the US has a safe and effective vaccine supply.
To learn more about vaccine safety, visit nfid.org/about-vaccines/safety and attend the Fall 2015 Clinical Vaccinology Course scheduled for November 13-15, 2015 in Bethesda, MD. For additional information, visit nfid.org/cvc.
To join the conversation, follow NFID (@NFIDvaccines) on Twitter, like NFID on Facebook, join the NFID Linkedin Group, and subscribe to NFID Updates.
Related Posts

The New Safety Question: “Is Your Child Vaccinated?”
With more parents delaying vaccination, this mom realized there is a safety risk when her children play with other kids whose vaccination status is unknown
