
Annual influenza (flu) vaccination is recommended for everyone age 6 months and older as the best way to prevent influenza (flu) and related complications. Flu vaccines are reviewed annually and updated as needed to protect against the influenza viruses that research indicates will be most common during the upcoming season. Even in cases when vaccination does not prevent infection, it can reduce the severity of disease and prevent serious flu-related complications, including hospitalization and death.
There are several types of flu vaccines offered at many convenient locations, including physician offices, public health departments, pharmacies and retail stores, workplaces, and schools. Many insurance plans pay for annual flu vaccination. Individuals covered by Medicare Part B can get a flu vaccine at no cost (no co-pay, no deductible). Health experts advise not to delay getting a flu vaccine if your first choice of vaccine is not available.
What types of flu vaccines are available?
The Centers for Disease Control and Prevention (CDC) recommends the use of licensed, age-appropriate flu vaccines. All flu vaccines available in the US for the 2023-2024 season are quadrivalent vaccines, which are designed to protect against 4 different flu viruses—2 influenza A viruses and 2 influenza B viruses. Options include inactivated influenza vaccines [IIV4], recombinant influenza vaccine [RIV4], and live attenuated influenza vaccine (LAIV4).
Different vaccines are licensed for different age groups, and some vaccines are not recommended for certain groups of people. Three flu vaccines are preferentially recommended for adults age 65 years and older:
- Quadrivalent high-dose inactivated flu vaccine
- Quadrivalent recombinant flu vaccine
- Quadrivalent adjuvanted inactivated flu vaccine
If none of these 3 preferentially recommended flu vaccines is available, adults age 65 years and older should get any other age-appropriate flu vaccine instead.
New egg allergy recommendations for 2023-2024: The influenza A(H1N1)pdm09 vaccine virus component was updated for egg-based and cell- or recombinant-based flu vaccines. It is no longer recommended that people with severe allergy to eggs be vaccinated in specific settings. All vaccines should be given in settings where allergic reactions can be recognized and treated quickly. People with egg allergy may get any vaccine (egg-based or non-egg-based) that is otherwise appropriate for their age and health status.
Flu vaccines available in the US include:
- Standard-dose flu vaccines without adjuvant, manufactured using virus grown in eggs, and approved for individuals age 6 months and older, as an intramuscular injection
- Cell-based standard-dose flu vaccine without adjuvants, which contains virus grown in cell culture, and approved for individuals age 6 months and older, as an intramuscular injection
- Inactivated flu vaccines preferentially recommended for adults age 65 years and older, as an intramuscular injection:
- High-dose, egg-based influenza vaccine (which has 4 times the antigen compared with a standard-dose inactivated influenza vaccine)
- Standard-dose, adjuvanted egg-based influenza vaccine
- Recombinant flu vaccine (made without influenza viruses or eggs), approved for individuals age 18 years and older as an intramuscular injection, and 1 of the 3 preferentially recommended flu vaccines for adults age 65 years and older (this vaccine has 3 times the antigen compared with a standard-dose inactivated flu vaccine)
- Live attenuated influenza vaccine (LAIV4), a nasal-spray vaccine made with attenuated (weakened) live flu viruses, approved for use in individuals age 2 years through 49 years. This vaccine is not recommended for use in pregnant women or in individuals with certain medical conditions.
Flu vaccines should be used with caution in anyone with a history of Guillain-Barré Syndrome within 6 weeks following a previous flu vaccine dose.
Flu and COVID-19:
COVID-19 vaccines and flu vaccines may be administered at the same time. Data are limited on coadministration of flu vaccines with recently introduced respiratory syncytial virus (RSV) vaccines. CDC General Best Practice Guidelines for Immunization support giving all vaccines for which a patient is eligible during the same visit.
Flu vaccines are not designed to prevent COVID-19 infection, and COVID-19 vaccines are not designed to prevent flu infection. Both vaccines must be received to help provide protection against both of these potentially serious respiratory infections.
For current guidance, see Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices — United States, 2023–2024 Influenza Season. MMWR Recomm Rep 2023;72(No. RR-2):1–25. DOI: http://dx.doi.org/10.15585/mmwr.rr7202a1
Reviewed September 2023
Source: Centers for Disease Control and Prevention
Related Resources
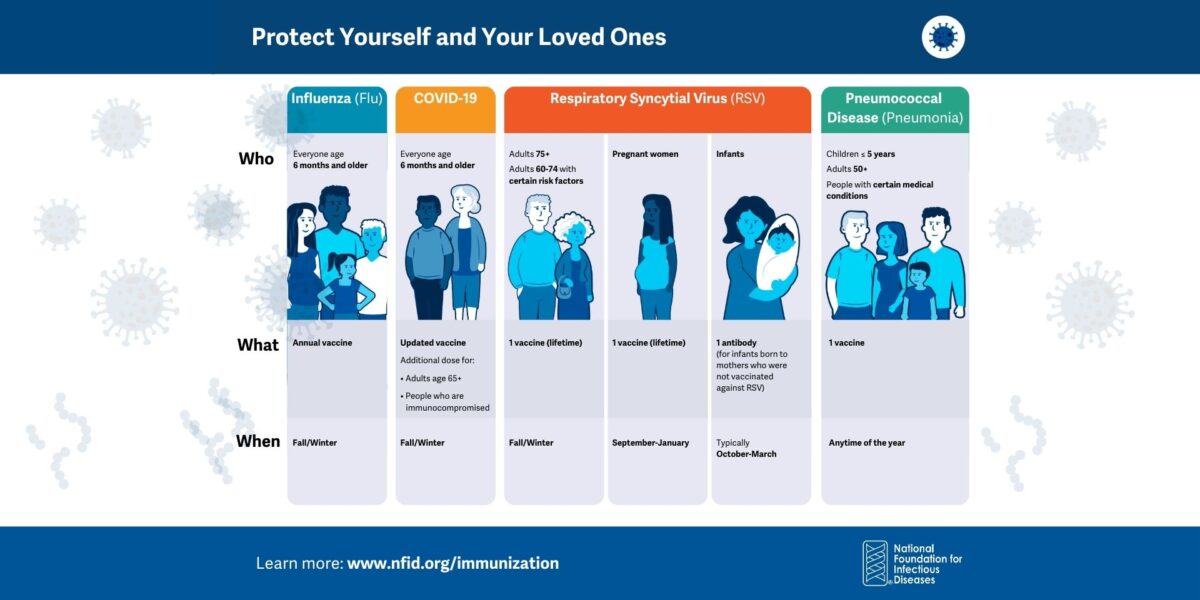
Respiratory Immunization Graphics
Graphics and sample social posts to help raise awareness about preventing COVID-19, flu, RSV, and pneumococcal disease

2024 National Survey: Attitudes and Behaviors about Influenza, COVID-19, Respiratory Syncytial Virus, and Pneumococcal Disease
2024 national survey on flu, RSV, COVID-19, and pneumococcal disease

Updated Recommendations for Respiratory Season
In this episode, NFID experts discuss updated recommendations to help protect against COVID-19, influenza (flu), respiratory syncytial virus (RSV), and pneumococcal disease …
