In the US, all vaccines must be approved or licensed by the Food and Drug Administration (FDA), after which every vaccine is evaluated by an independent group of experts who review the trial data and recommend how the vaccine should be used.
This site reflects US immunization policies, including links to Centers for Disease Control and Prevention (CDC) immunization schedules.
CDC Recommendations
CDC RECOMMENDATIONS
Parent-friendly immunization schedule for infants and children from birth through age 6 years
CDC RECOMMENDATIONS
Spanish version of parent-friendly immunization schedule for infants and children from birth through age 6 years
CDC RECOMMENDATIONS
CDC recommended immunization schedules from birth through age 18 years
CDC RECOMMENDATIONS
CDC recommended immunization schedules for adolescents age 7-18 years
CDC RECOMMENDATIONS
Spanish version of CDC recommended immunization schedules for adolescents age 7-18 years
CDC RECOMMENDATIONS
CDC recommended immunization schedules for adults age 19 years and older
Vaccine Safety
Advisory Committee on Immunization Practices
The Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP) is an independent group of experts who review safety and efficacy data to make recommendations for the use of vaccines in the US, including schedules regarding the appropriate timing, dosage, and contraindications.
ACIP includes ex-officio members from other federal agencies and non-voting representatives of liaison organizations that bring related immunization expertise.
ACIP develops recommendations for the routine administration of vaccines, along with schedules regarding the appropriate timing, dosage, and contraindications.

Recommended Vaccines by Age Infographics
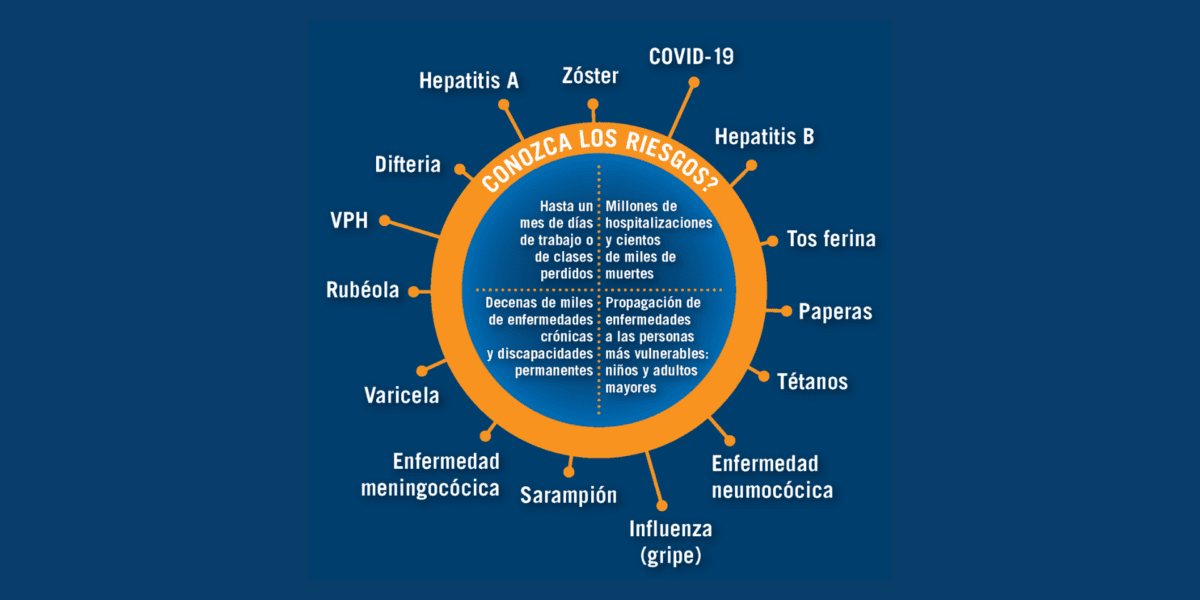
Recommended Vaccines By Age (Spanish)
Infographics in Spanish of vaccines recommended for children, adolescents, adults, and adults 65+, and the risks of vaccine-preventable diseases
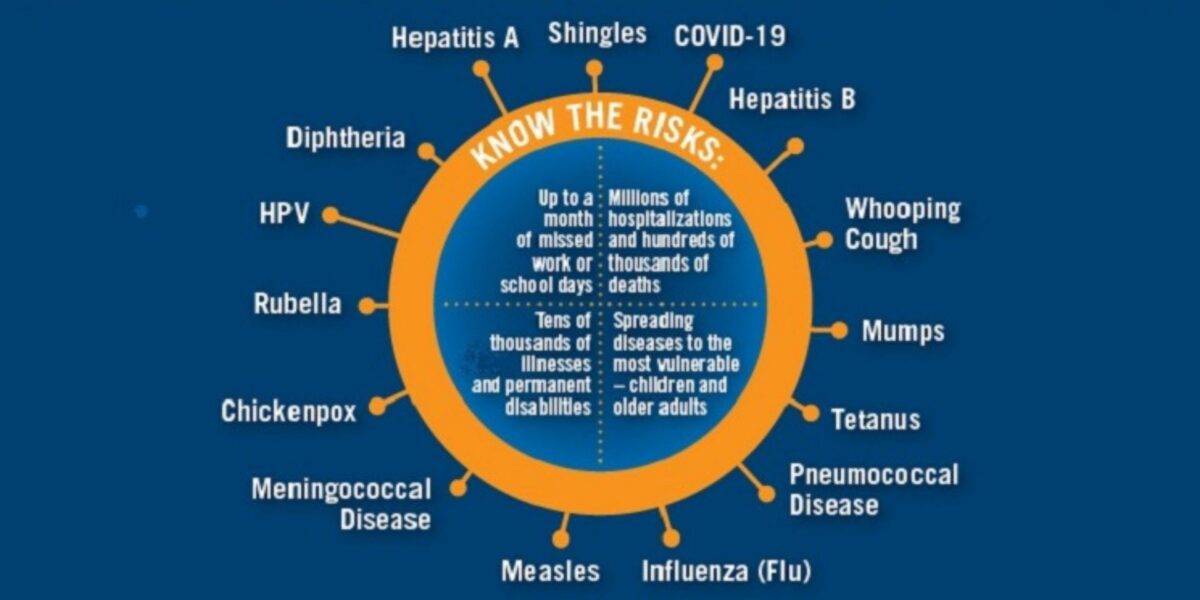
Recommended Vaccines By Age (English)
Infographics of vaccines recommended for children, adolescents, adults, and adults 65+, and the risks of vaccine-preventable diseases
Latest Posts
Statement on June 2025 Advisory Committee on Immunization Practices Meeting
NFID strongly supports the use of evidence-based guidance reviewed by qualified experts, within CDC and externally, to inform public health policy, and will work to protect the health of people across the US …
Updated February 2023
Source: Centers for Disease Control and Prevention