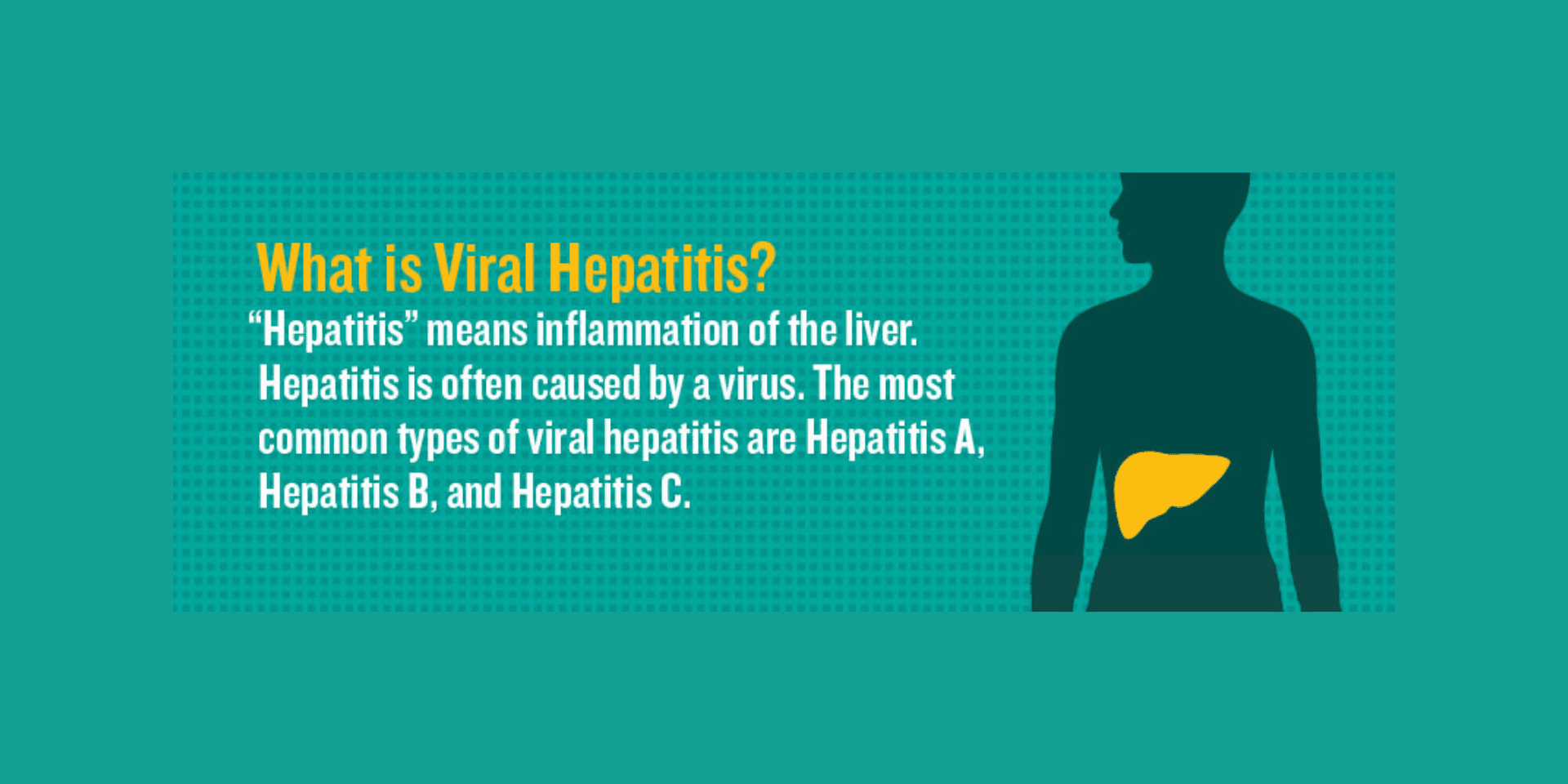
Over the past 15 years, the use of hepatitis A and B vaccines as recommended by the Advisory Committee on Immunization Practices (ACIP) has resulted in a substantial reduction of cases of both types of viral hepatitis. In the US, an estimated 850,000-2.2 million individuals are chronically infected with the hepatitis B virus and each year, approximately 30,000-50,000 cases of hepatitis A occur. New cases of hepatitis B infection in the US had been decreasing until recently; however, in recent years, acute cases of hepatitis B have increased and there have been several outbreaks of hepatitis A.
This past year, two further concerns became evident: 1) under the influence of the national epidemic of opioid abuse, rates of hepatitis B in middle-aged adults actually started to rise and 2) outbreaks of hepatitis A have occurred in several US cities, often among homeless populations.
To help address the challenges surrounding hepatitis A and B in the US, the National Foundation for Infectious Diseases (NFID) hosted a webinar* in October 2017 and subsequently developed responses to frequently asked questions:
Hepatitis A
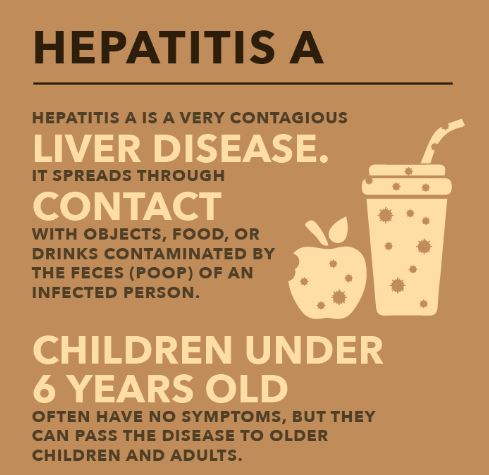
What is the recommended treatment for acute hepatitis A virus?
Unvaccinated individuals who have been exposed recently (within 2 weeks) to hepatitis A virus (HAV) should get hepatitis A vaccine or immune globulin to prevent severe illness. There is no specific treatment for hepatitis A. Supportive care, such as fluids, nutrition, and rest, is also recommended.
How strict is the recommendation to administer the end dose of hepatitis A vaccine at 6 months? Are there data to support administering a 3rd dose if the 2nd dose is administered within 6 months of the 1st dose?
A decreased immune response may occur when doses are administered earlier than the recommended interval. Doses of any vaccine administered ≥5 days earlier than the minimum interval or age should not be counted as valid doses and should be repeated as age appropriate. The repeat dose should be spaced after the invalid dose by the recommended minimum interval. For example, if the first and second doses of hepatitis A vaccine were administered less than 6 months apart, the second dose is invalid and should be repeated at least 6 months after the invalid second dose.
Is there a risk of reactivation of hepatitis A post-infection?
Reinfection of hepatitis A does not occur. Protective antibodies (IgG) develop in response to HAV infection and confer lifelong immunity. However, relapsing hepatitis A has been described as an atypical complication of hepatitis A virus infection.
Are there any current issues with hepatitis A vaccine supply on a national basis?
Yes, as of November 2017, in light of ongoing outbreaks of hepatitis A among adults in several US cities, the demand for adult hepatitis A vaccine has increased substantially over the past 6 months and vaccine supply to meet this unexpected demand in the US has become constrained. The Centers for Disease Control and Prevention (CDC) website provides information on vaccine supply and shortages. Note that current constraints do not apply to the pediatric hepatitis A vaccine supply.
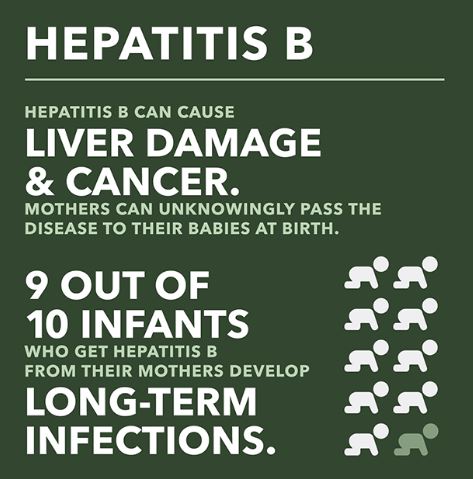
Why should infants be vaccinated against hepatitis B?
Approximately 90% of infants who are infected with hepatitis B develop chronic hepatitis B infection and about 1 out of 4 infected babies will die of liver failure or liver cancer as adults. All infants should be vaccinated in the national effort to completely eliminate mother-to-infant transmission of hepatitis B.
What is the recommendation on boosters and titers with hepatitis B for healthcare professionals?
Healthcare professionals (HCPs) who may come into contact with blood or body fluids during their work should be educated and offered vaccination against hepatitis B. Anti-HBs testing should be performed 1-2 months after administration of the last dose of the vaccine series. Completely vaccinated HCPs with anti-HBs <10 mIU/mL should receive an additional dose of hepatitis B vaccine, followed by anti-HBs testing 1-2 months later. HCPs whose anti-HBs remains <10 mIU/mL should complete the second series (usually 6 doses total), followed by repeat anti-HBs testing 1-2 months after the final dose. Alternatively, it might be more practical for very recently vaccinated HCPs with anti-HBs <10 mIU/mL to receive the second complete series (usually 6 doses total), followed by anti-HBs testing 1-2 months after the final dose. CDC Guidance for Evaluating Health-Care Personnel for Hepatitis B Virus Protection and for Administering Postexposure Management contains additional information. Once the vaccination and post-vaccination testing are complete, there are no recommendations for further periodic testing to assess anti-HBs levels and there are no recommendations for routine boosting with hepatitis B vaccine.
For medical workers/students who present without written evidence of hepatitis B vaccine series, is the recommendation to titer or revaccinate (without a titer)?
HCPs lacking documentation of hepatitis B vaccination should be considered unvaccinated (when documentation for hepatitis B vaccine doses is lacking) or incompletely vaccinated (when documentation for some hepatitis B vaccine doses is lacking) and should receive additional doses to complete a documented 3-dose hepatitis B vaccine series.
Hepatitis (General)
Why are non-injection drug users at risk for hepatitis?
Individuals that prepare and use non-injection drugs are typically in settings where they may have lapses in personal hygiene which increases the likelihood of disease transmission via shared equipment, drugs, or close personal contact.
If a pediatric patient receives an adult dose of either hepatitis A or B, do they need to be revaccinated?
No, however if the vaccine series is not complete, that individual should receive an age-appropriate dose at the next recommended interval.
*NFID Webinar (CME/CNE): Hepatitis A and B Vaccines: Recommendations and Impact. Presented by Noele P. Nelson, MD, PhD, MPH, Medical Officer in the Division of Viral Hepatitis at the Centers for Disease Control and Prevention (CDC). The webinar provides information on the immunogenicity and safety of hepatitis A and B vaccines, current ACIP recommendations, and the impact of vaccine implementation on the changing epidemiology of hepatitis A and B diseases.
To join the conversation, follow NFID on Twitter, like us on Facebook, follow us on Instagram, join the NFID Linkedin Group, and subscribe to NFID Updates.
Related Posts

Flawed ACIP Process Leads to Confusion and Distrust
Public health experts and leading healthcare professionals share concerns regarding the June 2025 Advisory Committee on Immunization Practices (ACIP) meeting on US immunization policy …

Empowering Men to Prioritize Health
Staying up to date on all recommended vaccines and taking other steps to prevent illness helps ensure men are ready for what matters most—showing up for loved ones or simply enjoying life …

Autism and Vaccines: What the Science Really Says
Progress is being made in the search for the causes of autism, and this information may be valuable for families who are hesitant about vaccines