A vaccine is developed each year to protect against circulating flu virus strains and every year, millions of people get an influenza (flu) vaccine. Unfortunately, it’s not possible to predict with certainty which viruses will circulate during the upcoming season since the flu virus has a tendency to mutate and evolve from one season to the next. However, scientists are now closer to developing a universal flu vaccine that could provide protection against a broad range of various influenza strains.
Rather than developing and manufacturing a new influenza vaccine each year, the more important need is to create a vaccine capable of providing long-term protection against many strains of the flu virus.
Although some parts of the flu virus evolve, there are other parts of the virus that remain stable year after year. These “parts” are also relatively the same between different strains of flu. Universal vaccines target the immune system at these relatively stable parts of the virus.
Within the past six months, two groups of researchers have independently created vaccines that lay the foundation for a long-anticipated vaccine that may protect against all types of flu viruses. A universal flu vaccine is now getting closer to reality.
Special thanks to Peter Palese, PhD and Teddy John Wohlbold (MD & PhD candidate) of the Icahn School of Medicine at Mount Sinai for this guest post featuring a deeper dive into the science behind the creation of a universal flu vaccine. Don’t miss Dr. Palese’s keynote presentation on Universal Flu Vaccines: Promise and Challenges at the 2016 Annual Conference on Vaccine Research scheduled for April 18-20, 2016 in Baltimore, MD.
 In a seemingly never-ending quest to evade human immunity, the influenza virus hemagglutinin (HA) antigen located on the surface of the virus constantly acquires mutations. These mutations allow the virus to escape neutralizing antibodies produced by the host, a process called antigenic drift. Thus, in contrast to vaccines that confer lifelong protection and rarely require booster immunizations (e.g., measles, mumps, and rubella (MMR) vaccine), influenza virus vaccines must be reformulated and re-administered on an annual basis to keep up with these changing mutations.
In a seemingly never-ending quest to evade human immunity, the influenza virus hemagglutinin (HA) antigen located on the surface of the virus constantly acquires mutations. These mutations allow the virus to escape neutralizing antibodies produced by the host, a process called antigenic drift. Thus, in contrast to vaccines that confer lifelong protection and rarely require booster immunizations (e.g., measles, mumps, and rubella (MMR) vaccine), influenza virus vaccines must be reformulated and re-administered on an annual basis to keep up with these changing mutations.
Every year, the US Food and Drug Administration decides which influenza virus strains will be included in licensed US vaccines. These considerations are based on surveillance data from the World Health Organization, which assesses the antigenicity of circulating influenza virus strains from 142 sites around the world in an attempt to predict which strains will antigenically match those that are anticipated to circulate in the upcoming season. However, it is currently impossible to perfectly predict the antigenic changes of the influenza virus HA. Even when the vaccine strains are well-matched to strains circulating in the human population, the efficacy of trivalent inactivated vaccines in young healthy adults is estimated to be approximately 75%, at best. The need for a more effective vaccine, ideally a universal vaccine that protects against all strains, is clear.
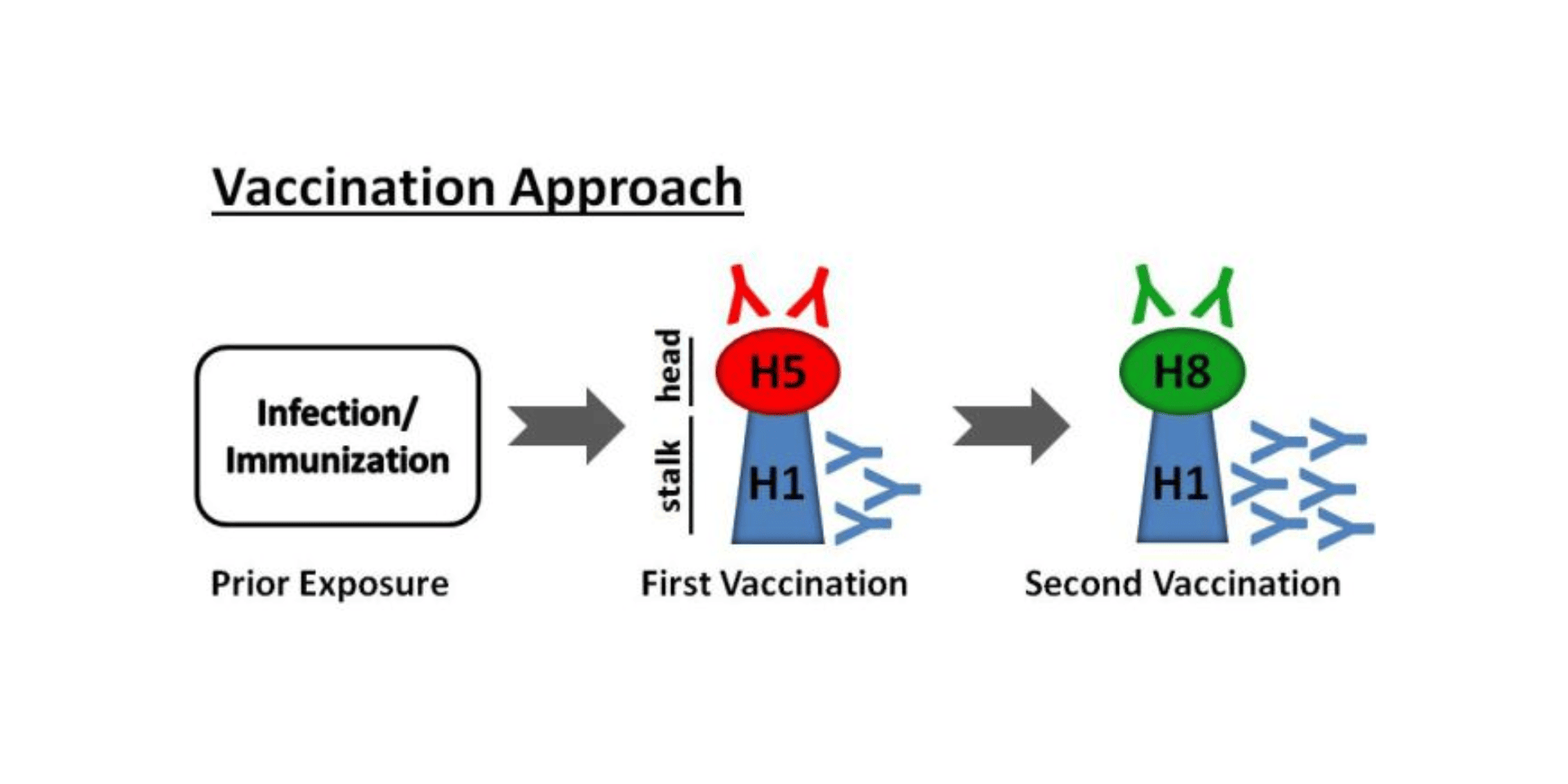
Our laboratory has generated an approach to developing a universal influenza virus vaccine that hinges on the unique structure of the HA protein on the surface of the virus. The HA protein is comprised of a head domain, which is very flexible and mutates easily, and a stalk domain. During influenza virus infection or vaccination, the human antibody response is predominantly directed to the head domain, and head-binding antibodies are highly strain-specific. In contrast, the HA stalk domain is structurally rigid and is largely the same across all strains. Antibodies against this region, called stalk-reactive antibodies, have been extensively characterized by our laboratory and by others, and have been shown to display tremendous breadth in binding across strains that have mutated. Such antibodies are protective against influenza infection in a variety of animal models, and are naturally generated to low levels in the human population.
To purposefully skew the immune response towards the stalk region of the HA, our laboratory has engineered HAs that possess the stalk domain of the strains that commonly circulate in the human population. Our approach aims to create vaccines to specifically boost antibodies against the stalk domain by sequential immunization. With each successive vaccination, the immune system is exposed to an HA with an identical stalk domain, and is coaxed to mount a greater immune response to the stalk domain (seen in the figure below). Such an approach has shown great promise in animal studies, protecting the animals against the influenza infection caused by varying influenza strains.
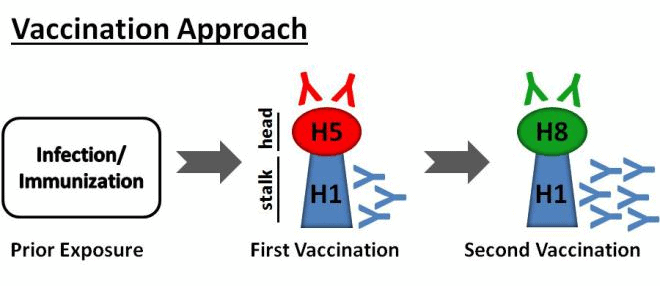
A universal influenza vaccine would be an exciting new advancement in the realm of influenza prevention, and would revolutionize a vaccination strategy that has remained largely unchanged for over 50 years. An attractive future is on the rise in which an individual would need to receive only two vaccinations over his or her lifetime to protect against the many commonly circulating influenza virus strains, as well as possible emergent pandemic strains. By further understanding the immunology of the stalk-reactive antibody response, our laboratory hopes to move one step closer towards the generation of a universal influenza virus vaccine.
To join the conversation, follow NFID on Twitter (@nfidvaccines) using the hashtag #ACVR, like NFID on Facebook, join the NFID Linkedin Group, and subscribe to NFID Updates.
Related Posts

Developing Improved Vaccines for Older Adults
By 2030, the number of adults age 65 years and older in the United States is expected to grow to 71 million—at least 20 percent of the total population. It is particularly important during this stage of life to maintain healthy lifestyles and habits. Receiving recommended immunizations is an essential part of that process.,,

A Forum for Cutting-Edge Vaccine Research Updates
The field of vaccinology continues to expand and innovate in basic science discovery, product development, market introduction, and adoption into immunization programs. Continual achievements are moving the field forward, with the expectation that many current, challenging diseases may become vaccine-preventable or vaccine-treatable in the near future…

Celebrating National Infant Immunization Week (NIIW)
The NFID 19th Annual Conference on Vaccine Research (April 18-20, 2016) organizers have developed a track of presentations and posters discussing maternal and infant immunization, in honor of National Infant Immunization Week.
