In the US, all vaccines must be approved or licensed by the Food and Drug Administration (FDA), after which every vaccine is continually evaluated for safety and efficacy. This site reflects evidence-based US immunization recommendations.

Recommendations by Age
Evidence-based immunization schedule for infants and children from the American Academy of Pediatrics (AAP)
Evidence-based immunization schedule for adults based on age and medical conditions from the American Academy of Family Physicians (AAFP)
Latest Posts
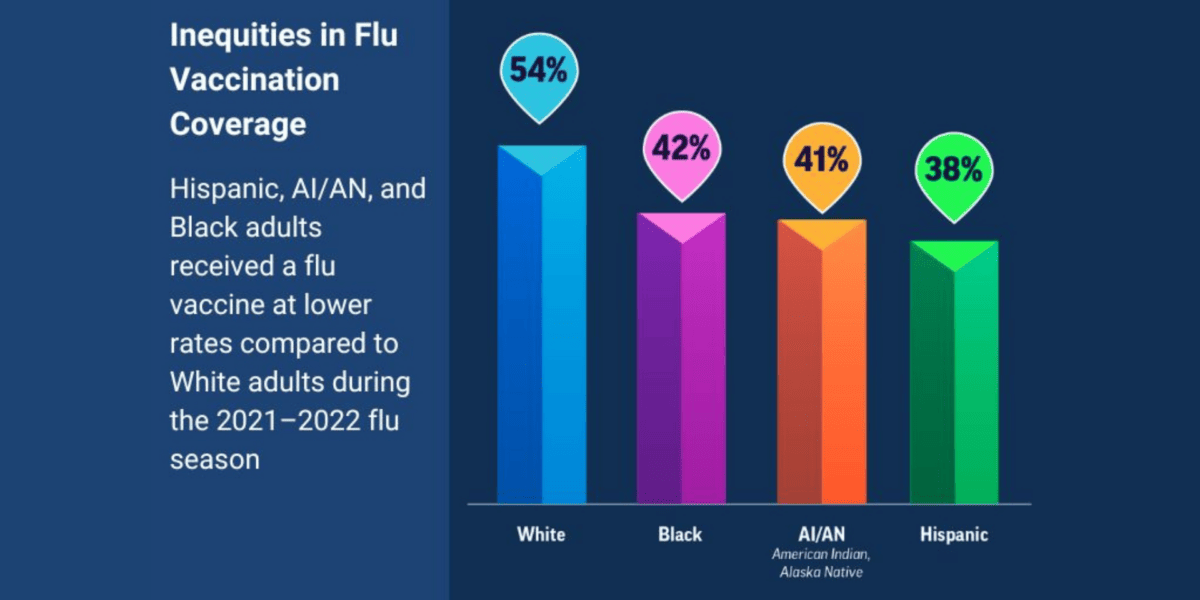
US Health Officials Urge Vaccination To Help Protect Against a Potentially Severe Flu Season
Following a mild flu season in 2021-2022, an NFID survey shows only 49% of US adults plan to get a flu vaccine this season
Updated August 2025
Sources: American Academy of Pediatrics, American Academy of Family Physicians